
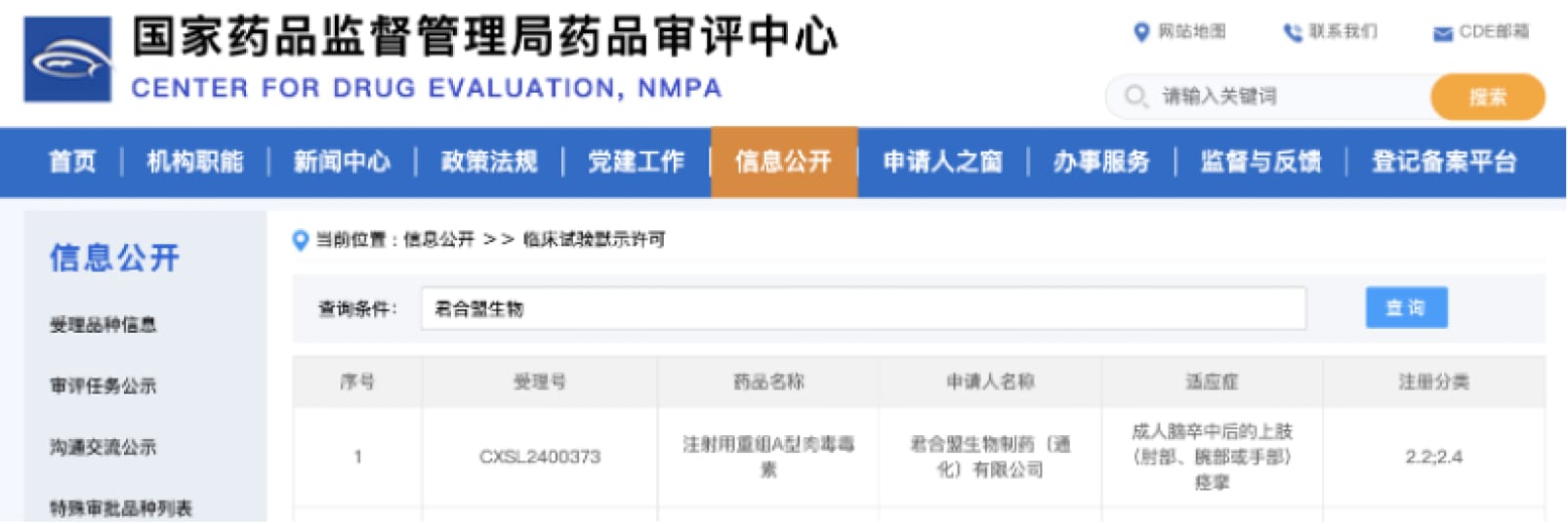
JHM Biopharmaceutical (Hangzhou) Co., Ltd. ("JHM") today announced that it has received approval from the National Medical Products Administration to begin a clinical trial of its injectable Recombinant Type A Botulinum Toxin for the treatment of upper limb spasticity in adult stroke patients.
 JHM Biopharmaceutical Receives Approval for Clinical Trial of its Recombinant Type A Botulinum Toxin for Stroke Spasticity
JHM Biopharmaceutical Receives Approval for Clinical Trial of its Recombinant Type A Botulinum Toxin for Stroke SpasticityJHM Biopharmaceutical (Hangzhou) Co., Ltd. ("JHM") today announced that it has received approval from the National Medical Products Administration to begin a clinical trial of its injectable Recombinant Type A Botulinum Toxin for the treatment of upper limb spasticity in adult stroke patients.
