JHM Biopharmaceutical has initiated the Phase III trial for recombinant botulinum neurotoxin of type A in treating upper limb spasticity in adults, with enrollment of the first patient completed.
Today, JHM Biopharmaceutical (Hangzhou) Co., Ltd. (hereinafter referred to as “JHM BIOPHARMA”) announce key progress in its independently developed injectable recombinant botulinum neurotoxin of type A. This product, manufactured and applied by its wholly-owned subsidiary JHM Biopharmaceutical (Tonghua) Co., Ltd., is intended for upper limb spasticity after stroke in adults. Its Phase III clinical trial has been approved by the National Medical Products Administration. This clinical trial-jointly led by Professor Fang Li, from Huashan Hospital of Fudan University, and Professor JingLing Jin, from Yangzhi Affiliated Rehabilitation Hospital of Tongji University (also known as Shanghai Sunshine Rehabilitation Center)-has officially commenced and completed the enrollment of the world’s first patient in the Phase III clinical trial of recombinant botulinum neurotoxin of type A for this indication, bringing a new breakthrough to this therapeutic area.

Figure: The Initiate Meeting of the First Phase III Clinical Research Center for Upper Limb Spasticity after Stroke in Adults
Dr. Chunsheng Leng, the Founder and Chief Scientist of JHM BIOPHARMA, stated:“We are highly confident that we can steadily advance the trial progress according to the research plan, to achieve the clinical transformation potential of this product as soon as possible, so that more patients can bendfit from high-quality and innovative biological treatment solutions.”
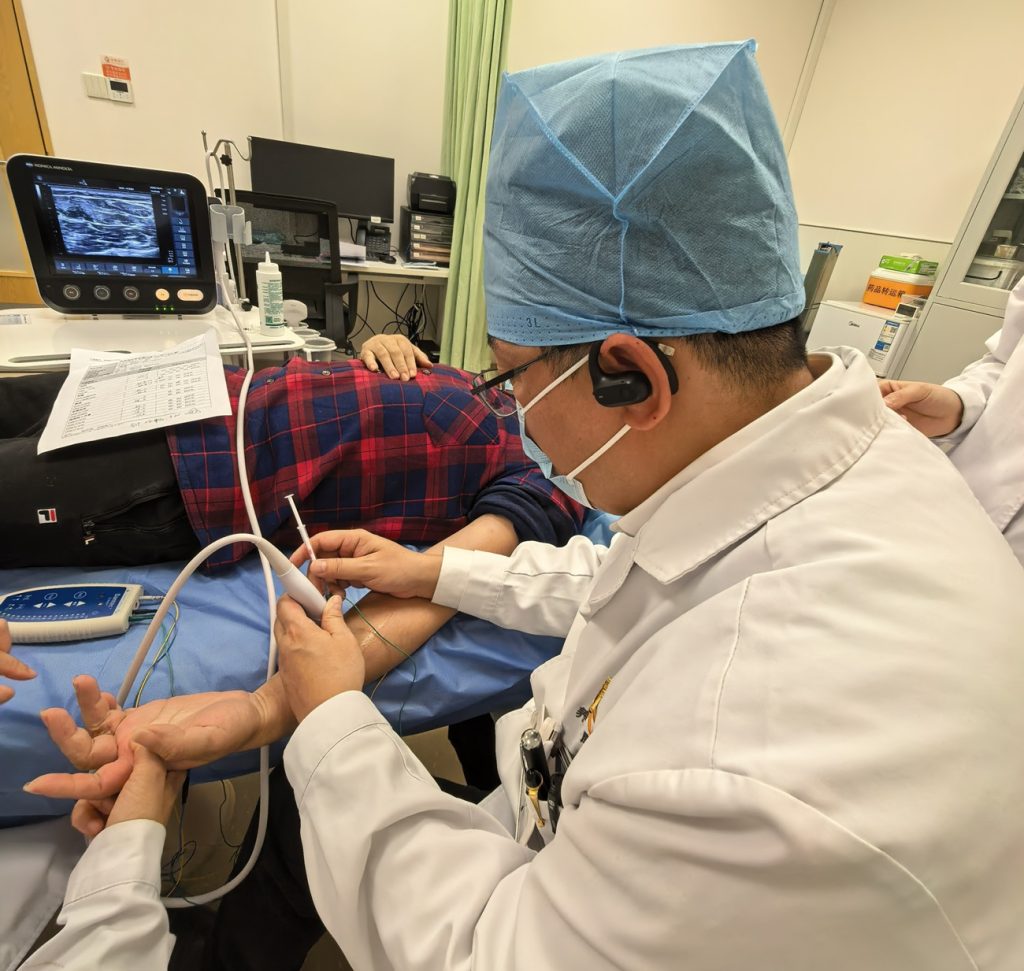
Figure: Professor Jin Lingjing, from Shanghai Yangzhi Rehabilitation Hospital, is administering the injection to the first participant.
This recombinant botulinum neurotoxin of type A was developed using genetic engineering, protein engineering and structural biology technologies, breaking through key technical barriers such as recombinant expression and formulation processes. It not only eliminates the biosafety risks in traditional preparation methods, but also demonstrates significant advantages in purity, activity and safety. It is expected to become an upgraded alternative to existing traditional botulinum toxin products.
As one of the first enterprises pioneering to master the core technology of recombinant botulinum neurotoxin of type A, the advancement of the Phase III clinical trial by JHM BIOPHARMA has not only established a critical foundation for its commercialization, but also poised to reshape the competitive landscape of the botulinum toxin market, promoting industry-wide advancements in safer, more controllable, and more efficient product development.
-The End-
About JHM Biopharmaceutical (Hangzhou) Co., Ltd.
JHM BIOPHARMA is an enterprise dedicated to the development and commercialization of innovative synthetic biology products and technologies. JHM is committed to advancing the use of innovative recombinant protein drugs in endocrine therapy, neurotherapy, and other next-generation biomedical material applications. Our mission is to provide groundbreaking biopharmaceutical products and treatments to patients worldwide. JHM BIOPHARMA portfolio includes recombinant botulinum neurotoxin of type A, human growth hormone injection, long-acting recombinant human growth hormone, single layer artificial dermis repair material (recombinant fully human), bilayer artificial dermis repair material(recombinant fully human), scaffold for cartilage repair(recombinant fully human).
Headquartered in Hangzhou, Zhejiang Province, JHM BIOPHARMA has established GMP-compliant manufacturing facilities in both Hangzhou and Tonghua. The enterprise possesses integrated capabilities spanning early drug discovery, process development, clinical research, manufacturing, and commercialization, giving it a competitive edge in driving rapid industry advancement and innovation. Since its inception, JHM BIOPHARMA has completed multiple rounds of financing, reflecting strong recognition from the capital markets regarding the enterprise’s technological expertise, product pipeline, and commercial potential. In 2024, the enterprise was designated as a “Specialized and Sophisticated SME in Zhejiang Province.”
